Neuromodulatory Systems and Behaviour
Our team has a broad interest in Systems Neuroscience. We are particularly interested in studying how learning is encoded in brain circuits, and how new behaviours that did not exist before imprint in neuronal networks when they are generated, matured and adapted upon changes in environmental rules. We focus our studies on the neuromodulatory processes supporting the subcortical brain networks that mediate voluntary behaviours, such as the striatum and the basal ganglia. We capitalise on basic learning theory, which allows us to generate accurate paradigms that expose very specific forms of learning in mice, and we deploy a series of quantitative fluorescence techniques as well as specific circuit tracing, recording and manipulation in behaving animals to explore causation. We are also interested in studying how these systems decline with age, and how this decline precipitates certain age-associated pathologies such as apathy, Parkinson’s disease and related cognitive disorders.
We focus on learning
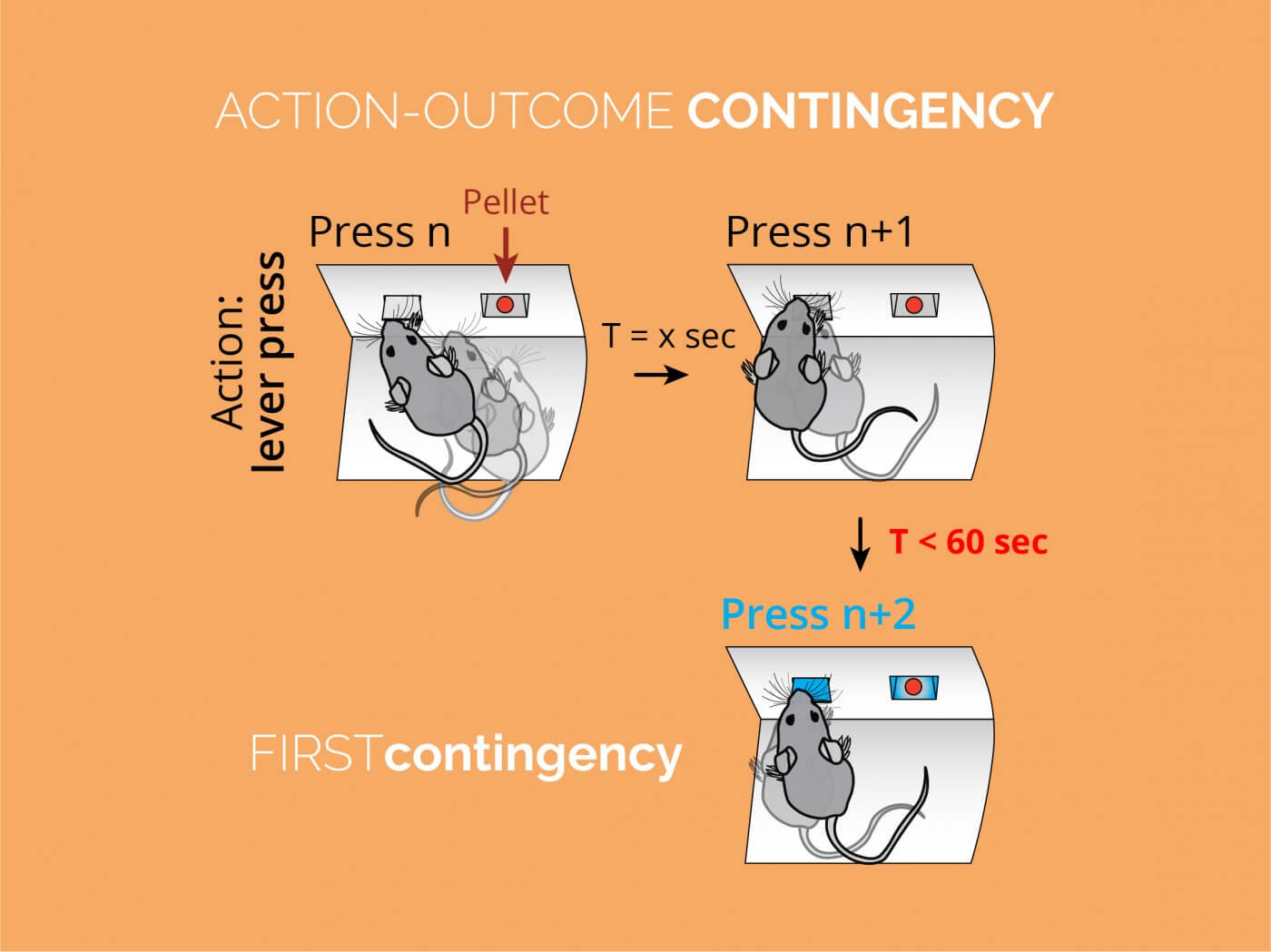
Classic learning theory provides researchers with powerful means to control behaviour. Associative events are primary processes in the brain that allow to generate and shape behaviours that ensure survival. Associative processes can also be created and maintained artificially in order to generate previously inexistent behaviours, or to control unwanted behaviours. In our lab, we take full advantage of classic animal learning theory, which allows us to expose the function of a particular brain circuit by bringing animals (i.e. mice and rats) to an acute conditioning state. Unlike other laboratories studying the basal ganglia, we centre our attention to the adaptations that the basal ganglia circuitry undergoes throughout the behavioural history, rather than focussing on one specific performance stage at one specific point in time. Our behavioural designs usually include variations of instrumental conditioning, but we also use paradigms with Pavlovian conditioning components or the combination of both. These methods are optimal to study how different neuromodulation processes capture and encode learning in the striatum.
The striatum: where neuromodulation explodes
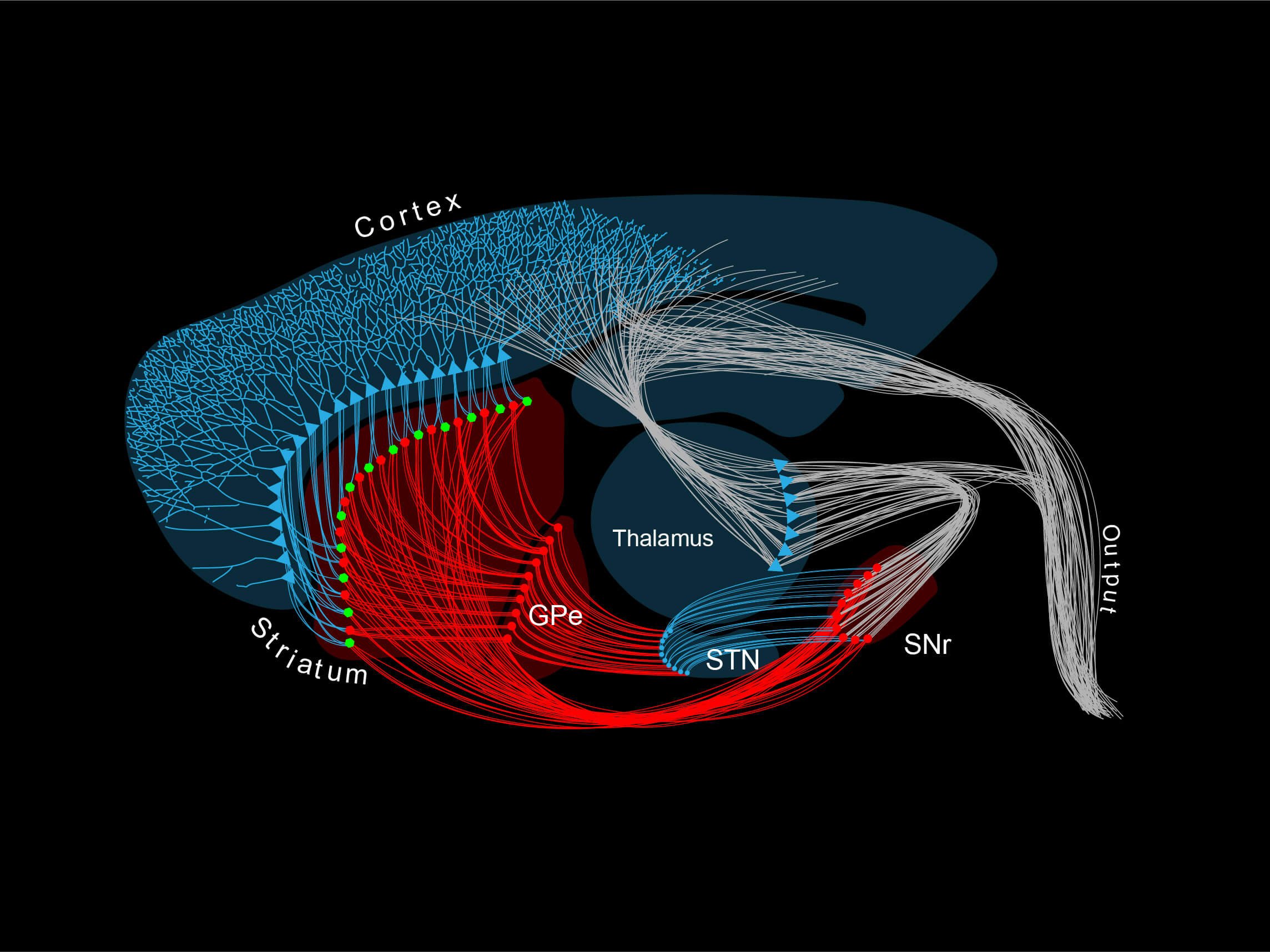
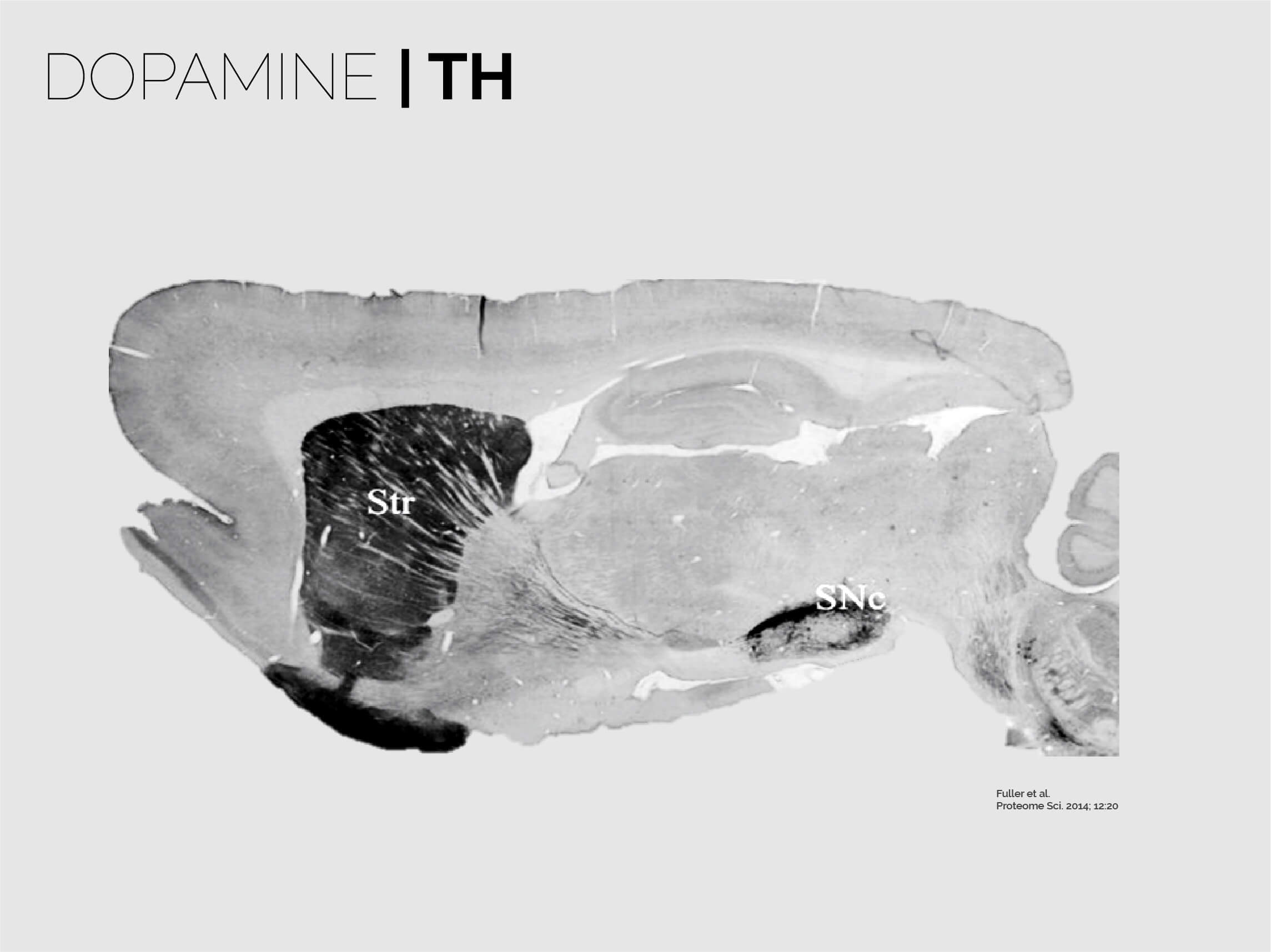
The striatum is the entry gate to the basal ganglia, a brain-scale closed loop circuit located at the core of the forebrain that centrifuges extensive intero- and exteroceptive information in order to generate fine behaviours that perfectly match the environment. As such, the neurons in the striatum sit at a frontier between glutamatergic and GABAergic worlds, and thus receive and process amongst the largest loads of neuromodulation in the brain. This includes dopamine, acetylcholine, neuropeptides, opioids, serotonin, adenosine and many others. Spiny Projection Neurons (SPNs), the main components of the striatum, are highly specialised to receive these signals, and to integrate them with the large corticostriatal and thalamostriatal drives. This step, as suggested by our research, is key to initially produce and continuously modify voluntary behaviour.
SPNs are a particular type of GABAergic neuron
SPNs are classically described as GABAergic projection neurons. However, they have several unusual features that make them special, beyond their feedforward inhibitory function. First, their dendrites are incredibly densely spined, consistent with their role in receiving large glutamatergic and neuromodulatory loads. Second, they are extremely active molecularly: they deploy large intracellular signalling programs that are usually triggered by coincident neuromodulatory and excitatory signals. Third, they themselves provide a source of neuromodulation, as their axonal fields extensively ramify locally, and they intrinsically release large quantities of different neuropeptides and modulators. All these features and more define SPNs as signal integrators and local neuromodulators, rather than simple synaptic conveyers.
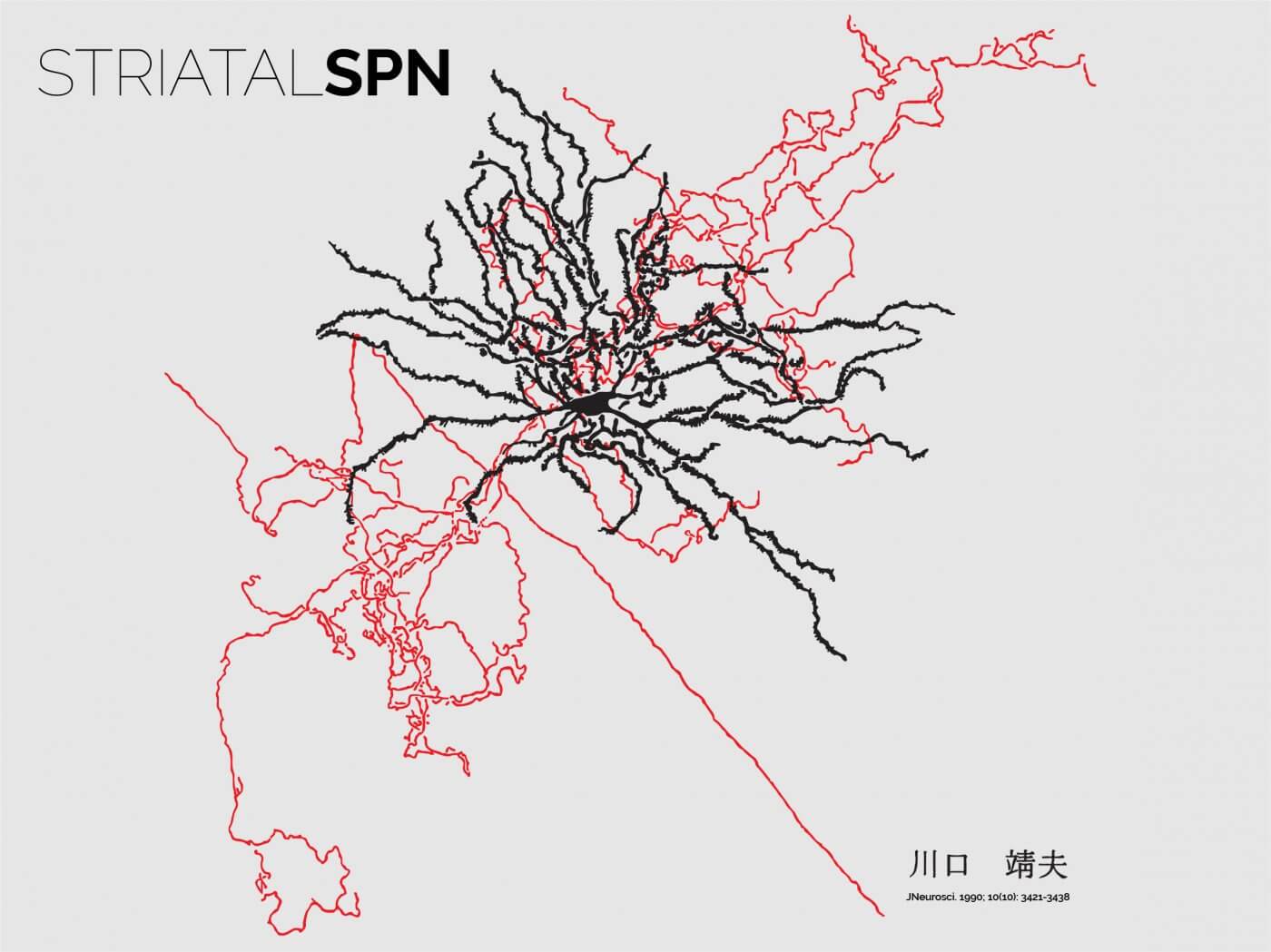
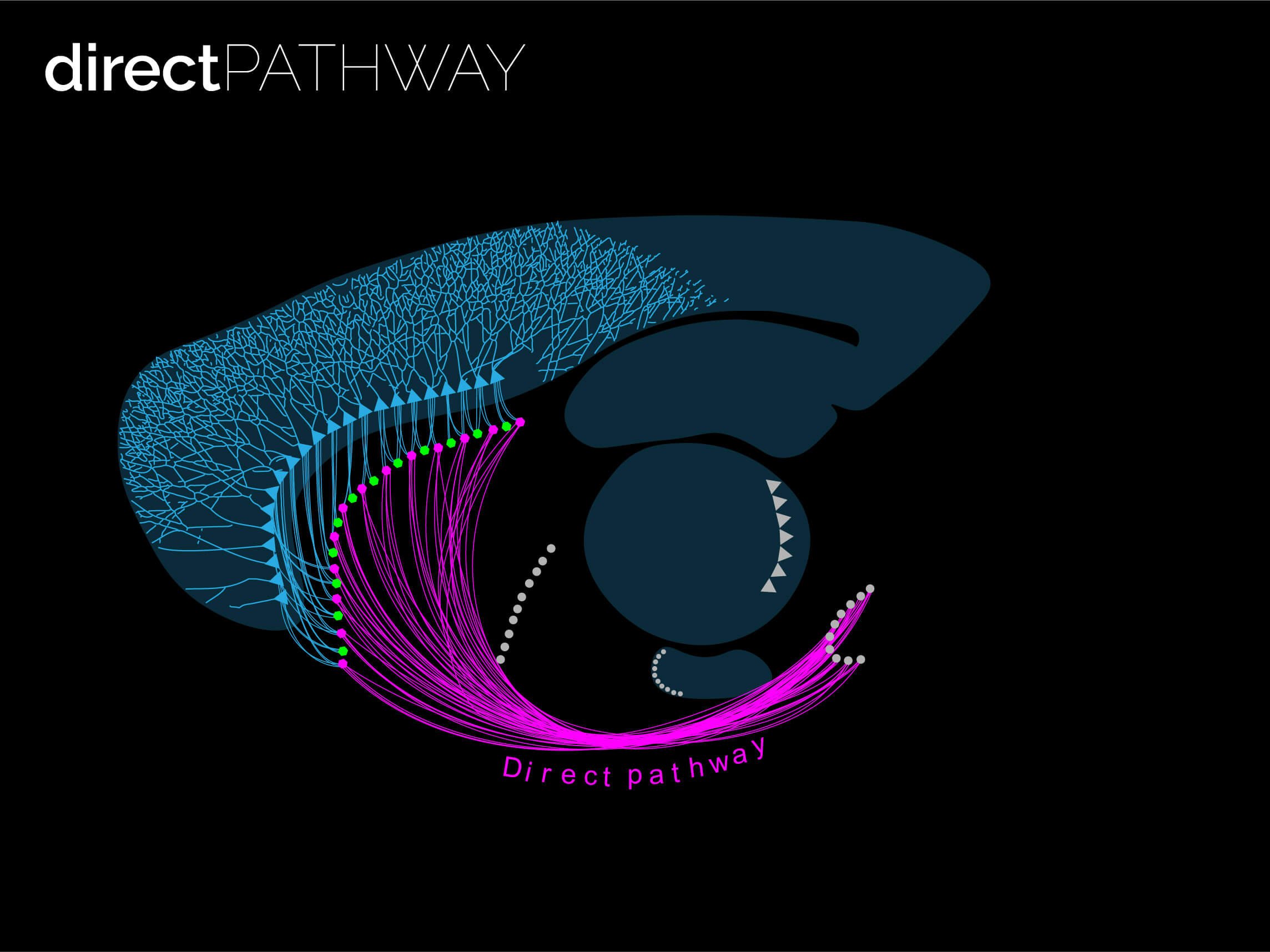
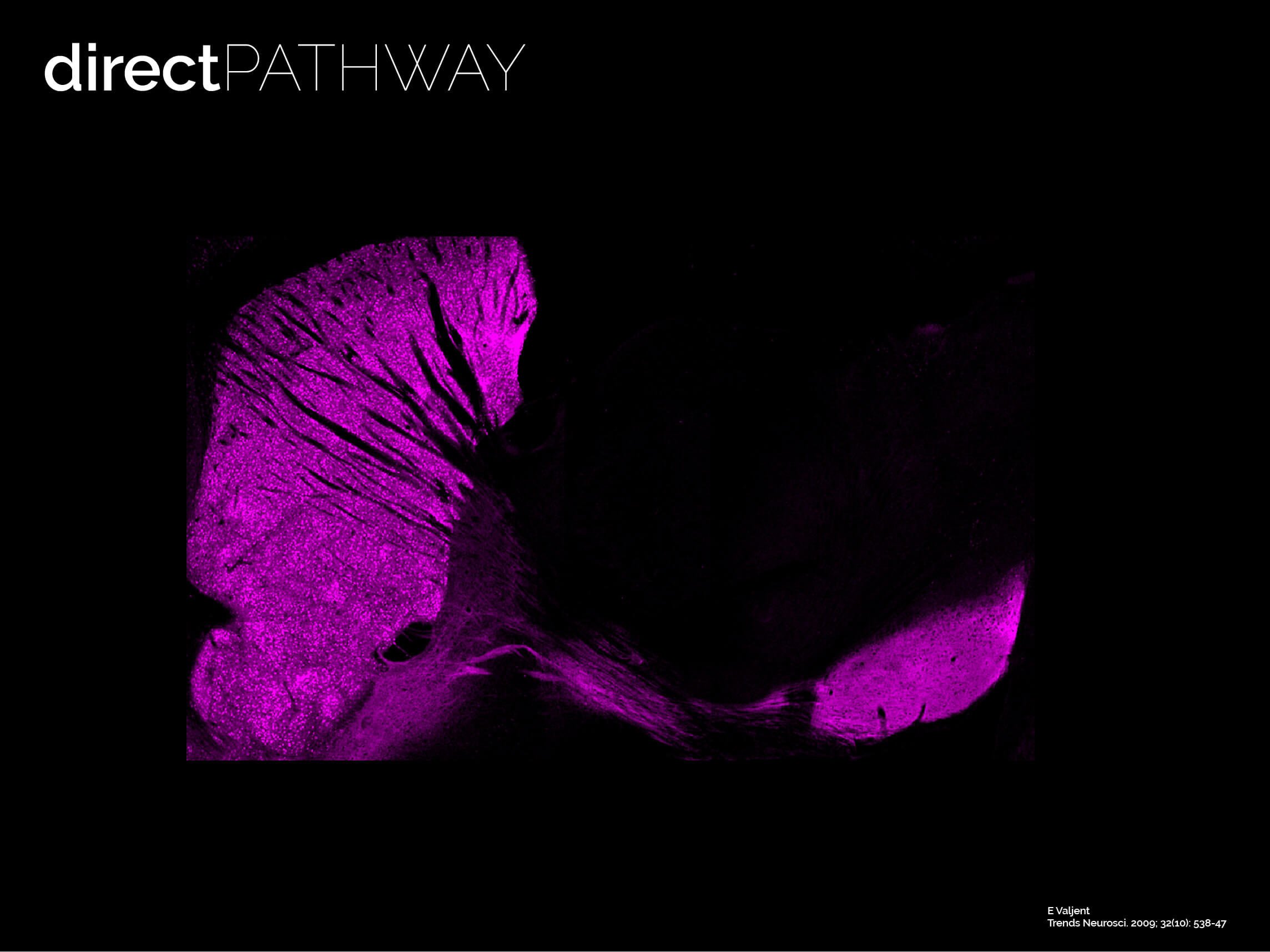
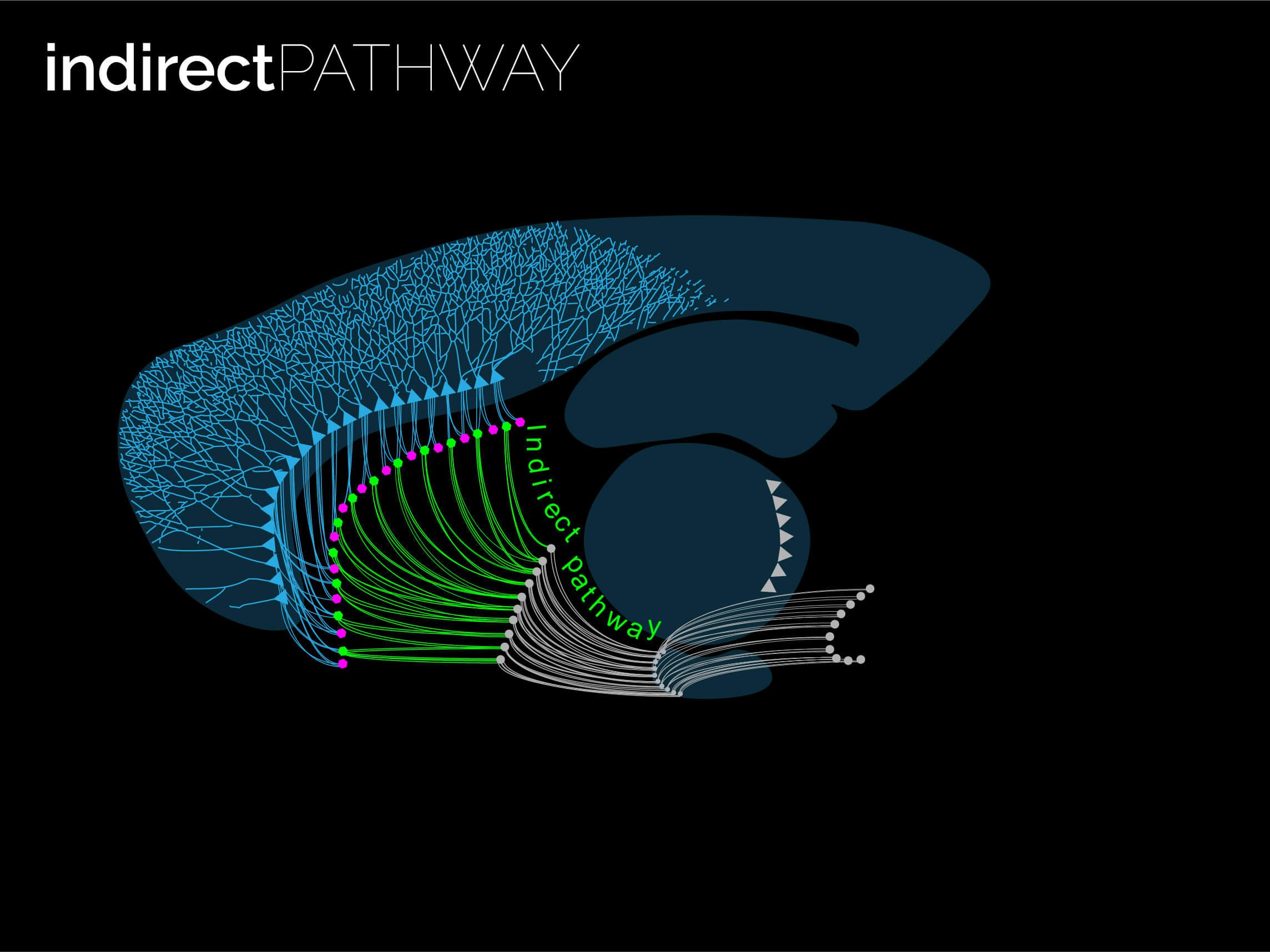
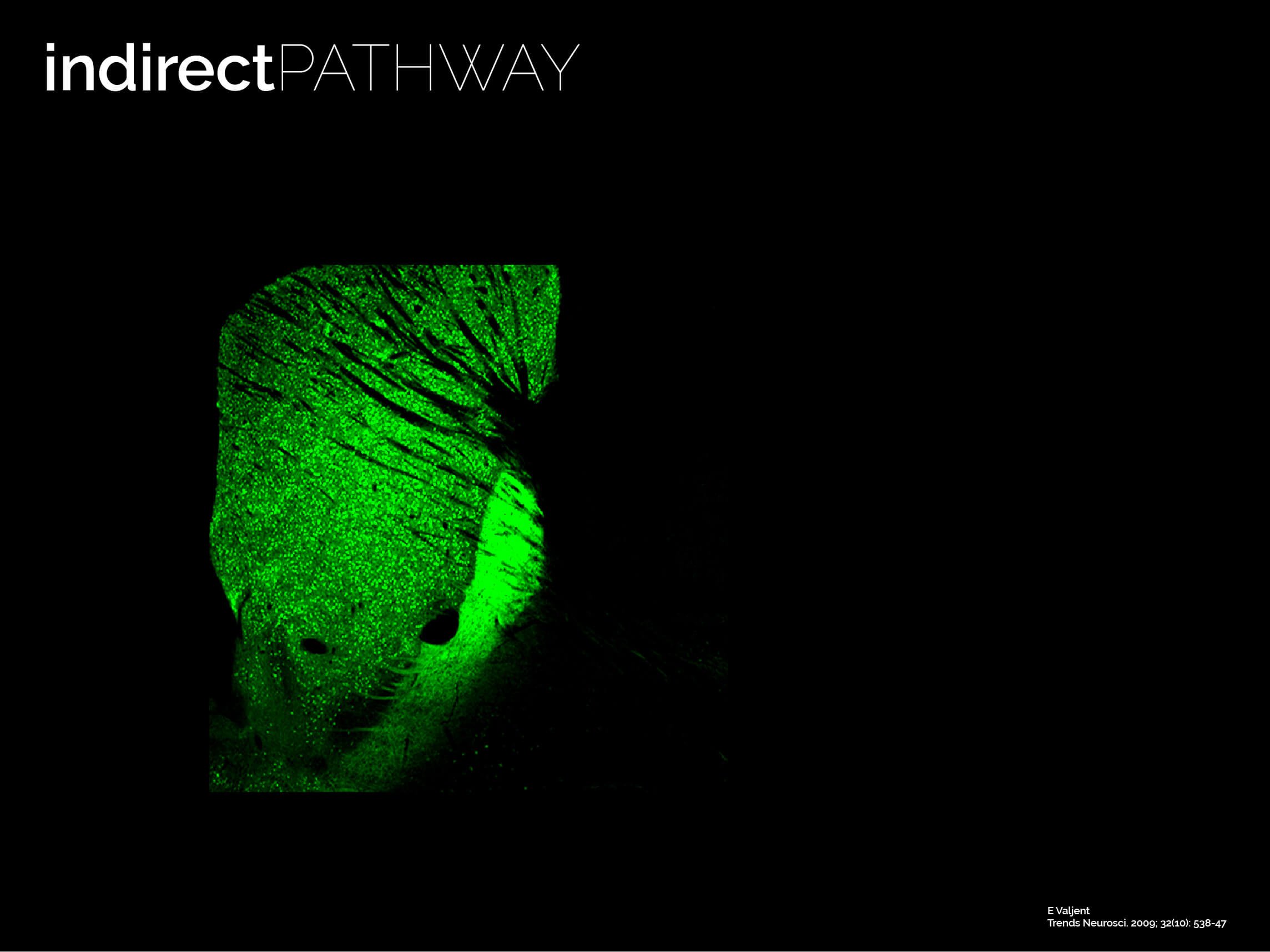
The twin neuronal systems in the striatum
The striatum is almost entirely composed by SPNs (> 95% of all neurons are SPNs). SPNs divide into two twin populations of similar proportions who have different neuromodulatory signatures and project to completely different targets in the basal ganglia. SPNs of the so called “direct pathway” (dSPNs) tend to provide a direct projection to the output of the basal ganglia, express specific sets of receptors for dopamine and acetylcholine, and release specific neuropeptides (e.g. dynorphin, Substance P). On the other hand, indirect pathway SPNs (iSPNs) exclusively project to pallidal structures and express particular sets of receptors for dopamine and adenosine, and also synthetise and release their own neuropeptides such as enkephalin. Why would there be two different populations?
The striatal binary mosaic
Both types of SPN are highly specialised to receive large loads of glutamatergic transmission, mostly from the corticostriatal and thalamostriatal projections but also from other centres in the brain. An enigmatic feature of SPN systems is that they are distributed in a random fashion, with no tissue structure such as layers or columns—which are commonly observed in other brain structures. There are no borders limiting the different functional territories, it basically resembles a sponge. Why? Even more puzzling is the fact that dSPNs and iSPNs are completely intermingled within the tissue, so both occupy all territories of the striatum at similar rates. How can such an unordered, homogenous environment have any specificity? How are functional domains established? Our research suggests that the striatum functions as a binary mosaic, where dSPN and iSPN systems interact with one another through bijective correspondence in a “one to one” fashion, and they do so by taking advantage of local neuromodulatory transmission.
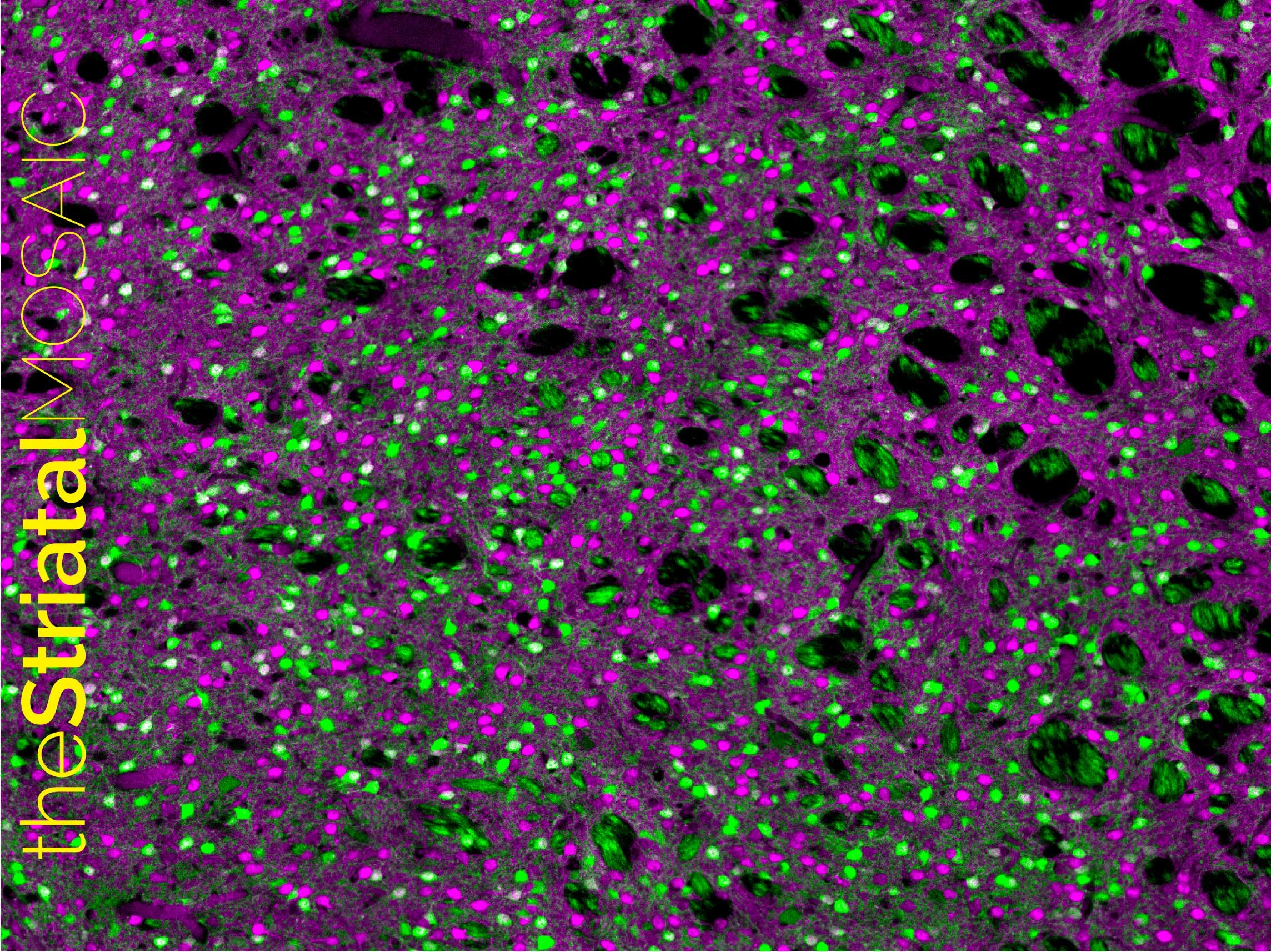
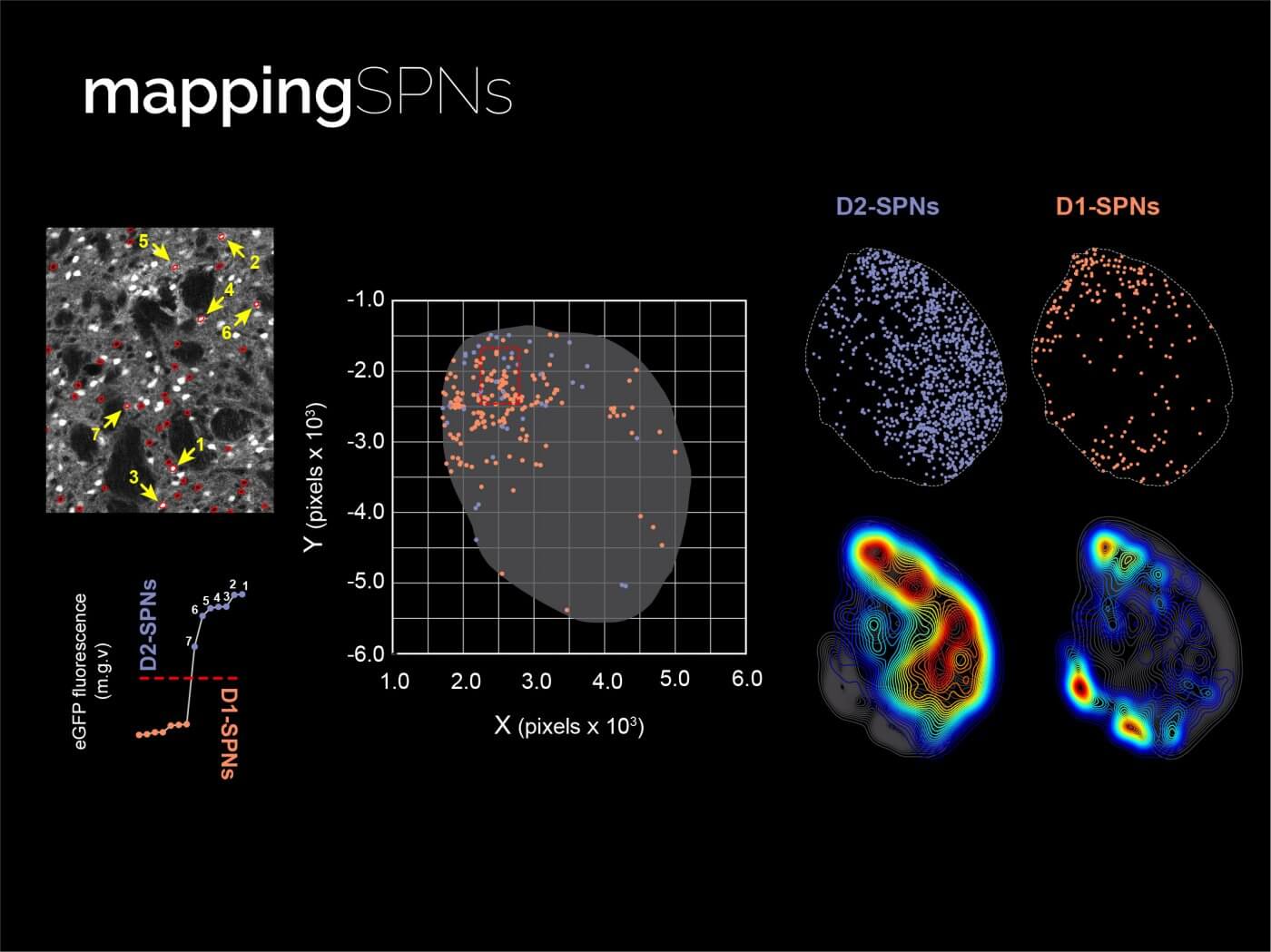
Mapping intracellular signalling in SPNs
One good way of seeing patterns of local modulation amongst SPN subtypes is to study how they behave molecularly during learning. In our lab, we have developed methods to map neurons that are at certain molecular signalling states by detecting different kinase activities in large expansions of the striatum. We combine this with transgenic animals that endogenously label specific neuronal populations, so we can phenotype each cell expressing signalling activity. We obtain maps of whole striatal sections where we can assess what regional pattern in each SPN type better characterises a specific learning state. We are also developing new strategies to measure neuromodulation and signalling events in real time, while animals perform behaviours.
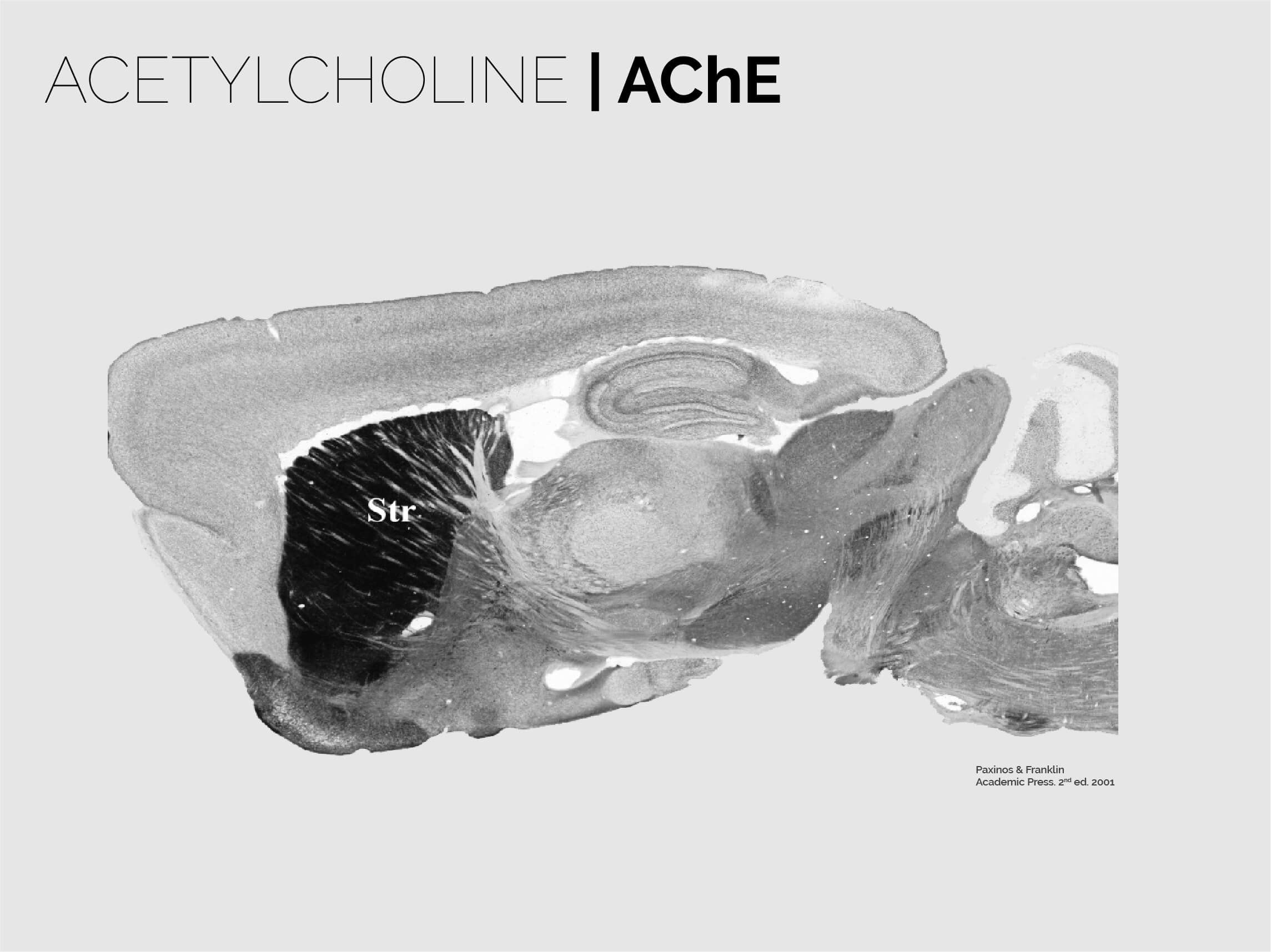
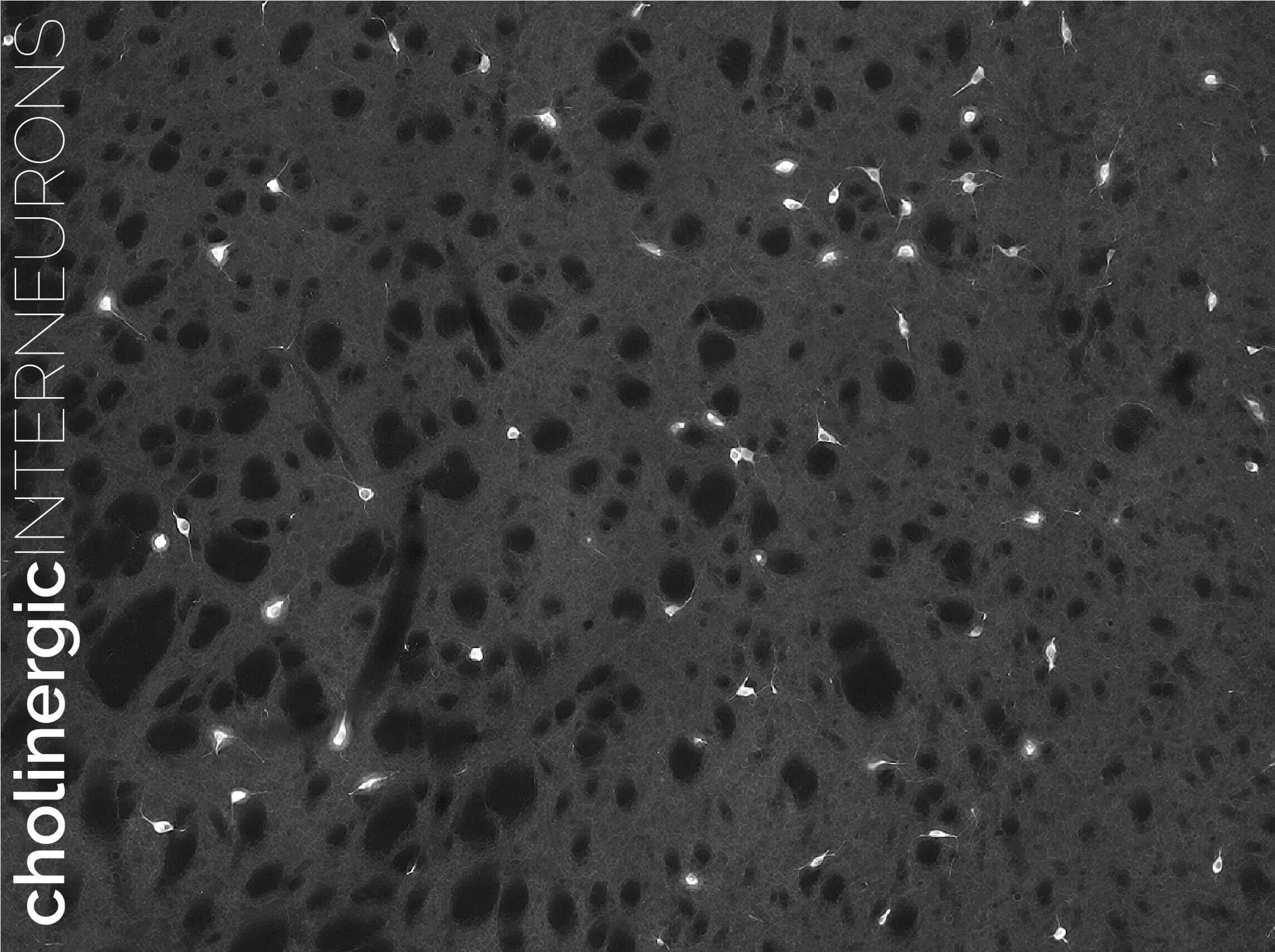
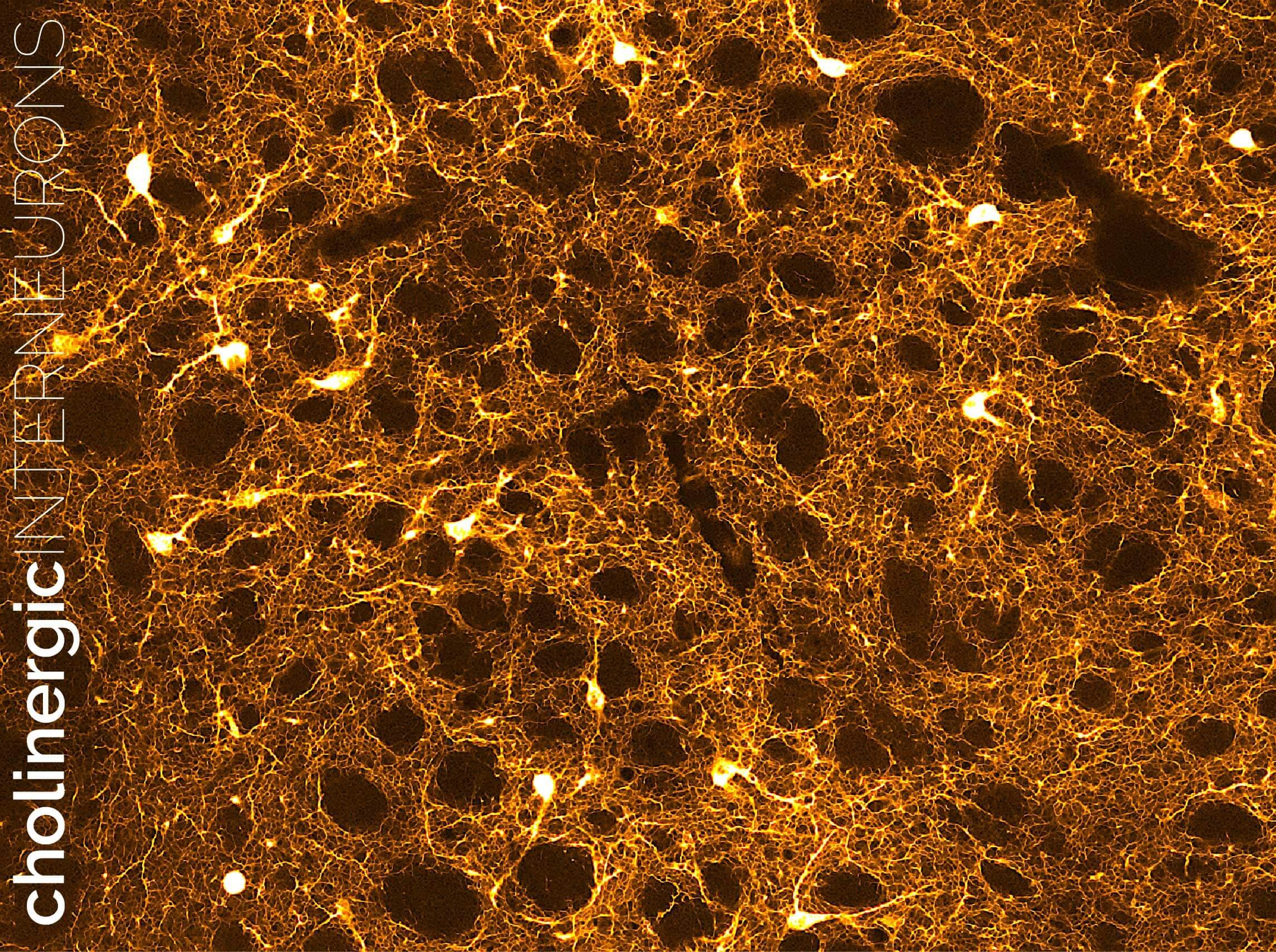
Modulating the modulators in learning: cholinergic interneurons
Cholinergic interneurons (CINs) are yet another intriguing system in the striatum. While only representing ~2% of the total neurons, they have huge somata, extensively branched dendrites and axons and, most importantly, are purposely spaced out in the striatal tissue. The result is a tile-like disposition that is perfectly suited to provide abundant and timely modulation to glutamate and dopamine release as well as SPN function. The way CINs mediate their functions in the striatum is a matter of intense research. Our lab has uncovered some key roles of CINs in predictive learning as well as the updating of previously acquired learning, and how this is linked to their inputs from specific regions in the thalamus. We are now studying the way these neurons interact with other neuromodulators in the striatum, and how they blend with the striatal binary mosaic to organise learning territories in the striatum.
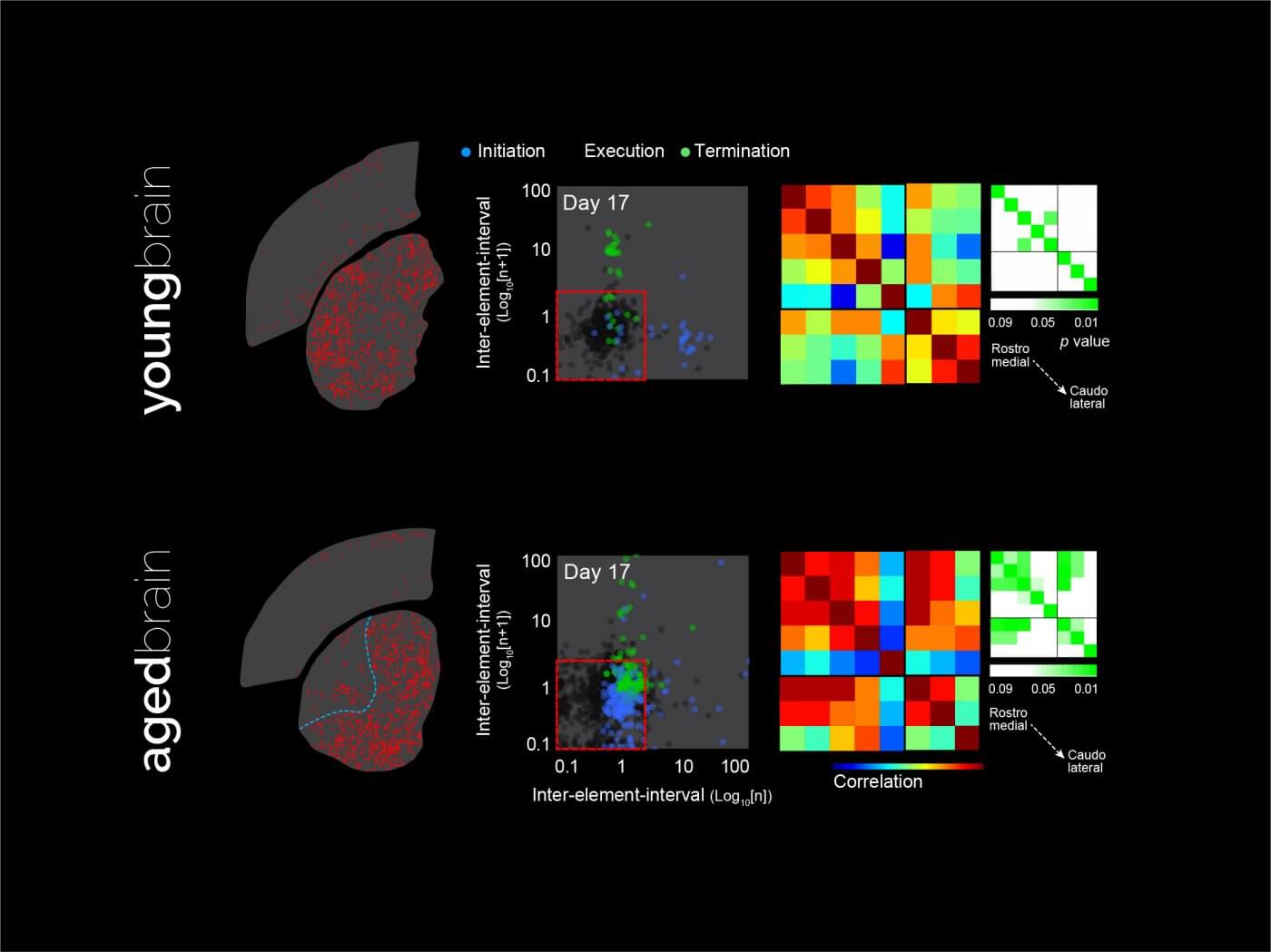
Ageing and the breakdown of the striatal mosaic
Normal ageing is usually accompanied by a serious reduction of motivated behaviour and goal-directed action. In our lab we are very interested in studying how striatal systems work in aged brains, who although not considered diseased, work differently. We have observed some very interesting characteristic regionalisation patterns in the striatum that differ to a large extent to what’s observed in the young brain, and that relates to aberrant learning of new behaviours. We hypothesise that age introduces some special features to the striatal mosaic that make it function in a completely different mode, perhaps due to engagement of compensatory urges that attempt to maintain compromised behavioural function. Identifying how brains change modes and why is pivotal to anticipate motivational decline and to define strategies to maintain striatal function.
GRANT FUNDING
Our work is currently supported by the Australian Research Council, the National Health and Medical Research Council, the Tourette Association of America and UNSW. Click for details
RESEARCH ETHICS & SAFETY
All experimental procedures are approved by the Animal Care and Ethics Commitee and the Gene Technology Research Commitee at UNSW.
CC LICENSE
Except where otherwise noted, NeuroModuLab.org by J.Bertran-Gonzalez & M. Matamales is subject to a Creative Commons Attribution 4.0 International license.
